Research
About Our Research
Our research is motivated by the way infectious diseases can cause wildly different outcomes in different people. What determines whether the same pathogen causes mild or severe disease in different people?
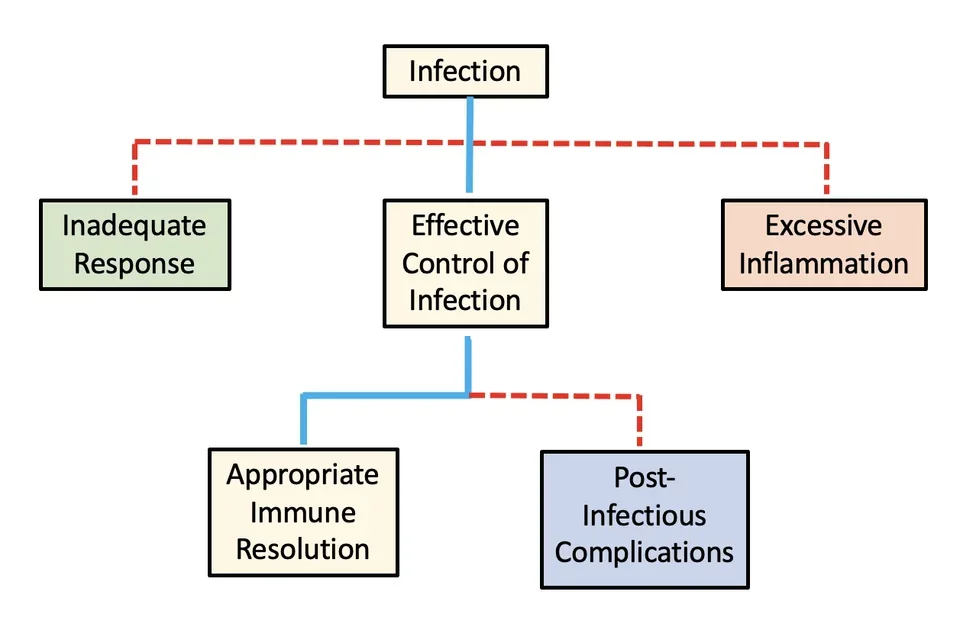
Innate immune cells are the front-line defense against infectious pathogens. How these cells respond to infection depends on their exposure history. In other words, for cells of the innate immune system, the past shapes the present. This is similar to how the human response to a given stimulus might differ depending on what had previously happened to us that day. If someone cuts us off on the freeway, we are much more likely to forgive the offense if we are coming back from a relaxing day at the beach than if we are coming off a stressful overnight work shift! Immune cells are no different. Context matters. The past shapes the response to present stimuli.
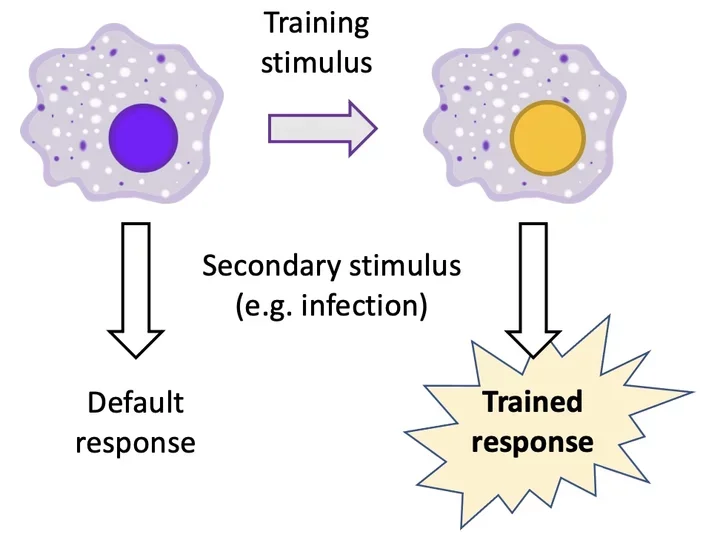
How past exposures reprogram cells of the innate immune system is known as “trained immunity.” Our research examines the molecular mechanisms of trained immunity and how it contributes to the variable outcomes of infections. Our hope is that by better understanding how trained immunity works we will be able to predict which patients are at risk for severe infections and provide our own “training” of the immune system to improve human health.
Current Research Topics
Stimulus-specificity of trained immunity
Innate immune cells (particularly monocytes and macrophages) are “trained” by past exposures. Interestingly, the effects of trained immunity are variable. In some cases, trained immunity primes the host to produce an increased inflammatory response to infection, but in other cases it diminishes the inflammatory response. Whether reprogrammed cells produce increased or diminished inflammatory responses depends on what stimulus forms the memory. That is, the effects of trained immunity are stimulus-specific. However, the mechanisms that account for this stimulus-specificity are unclear, and understanding these mechanisms will be critical for harnessing the therapeutic potential of trained immunity. In this project we are using a model of disseminated infection with the fungal pathogen Candida albicans to explore what molecular factors determine a given stimulus’ capacity to induce protective training.
Role of IRFs in epigenetic reprogramming
Trained immunity is associated with epigenetic changes. When immune cells encounter a training stimulus, they activate transcription factors such as NFκB or IRFs that can rearrange the DNA – opening chromatin and facilitating histone modifications – to activate previously silent regulatory DNA elements called latent enhancers. These newly active enhancers affect the gene expression response to subsequent infection. Our previous work [link] has focused on how NFκB activates latent enhancers, and our current work in collaboration with Alexander Hoffmann aims to understand how the IRF family of transcription factors produce epigenetic changes.
Context-dependent regulation of type I and III interferons
Interferons are cytokine molecules that are critical for proper immune response to infections, particularly viral pathogens. Because their effects are so potent, the production of interferons is tightly regulated by immune cells. In this project we are studying how past exposures affect the capacity of an immune cell to produce high levels of interferons. This has important implications for understanding why some people respond better than others to viral infections like Covid-19.
How chronic disease states reprogram human monocytes
Trained immunity can be induced not only by short-term exposures to acute stimuli but also by long-term exposures to chronic disease states. We are interested in how chronic conditions like diabetes, obesity, renal failure, or HIV-infection alter innate immune cell function. We are developing methods to profile the epigenetic state and the function of innate immune cells from patients with chronic diseases.